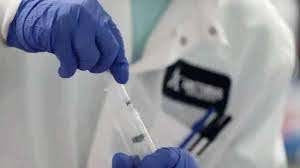
The Drug Controller General of India (DCGI) has granted approval for the commercial launch of India's first CRISPR COVID-19 test 'FELUDA'. The test has been developed by the Tata Group and CSIR-IGIB (Institute of Genomics and Integrative Biology). It is the world's first diagnostic test to deploy a specially adapted Cas9 protein to successfully detect the virus causing COVID-19.
The Drug Controller General of India (DCGI) has granted approval for the commercial launch of India's first CRISPR COVID-19 test 'FELUDA'. The test has been developed by the Tata Group and CSIR-IGIB (Institute of Genomics and Integrative Biology). It is the world's first diagnostic test to deploy a specially adapted Cas9 protein to successfully detect the virus causing COVID-19.









417.jpeg)
10.jpeg)
40.jpeg)
100.jpeg)
416.jpeg)
5.jpeg)
4.jpeg)
6.jpeg)
5.jpeg)





