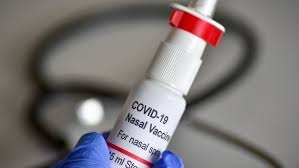
Hyderabad-based Bharat Biotech on Wednesday received "in principle" approval from Drug Controller General of India (DGCI) to test its intranasal COVID vaccine as booster shot. The Subject Experts Committee (SEC) of India's drug regulator DGCI granted "in principle" approval to Bharat Biotech to conduct "Phase III superiority study and Phase III booster dose study" trials for its intranasal COVID vaccine.









139.jpeg)
260.jpeg)
259.jpeg)
258.jpeg)





